Chapter 06. Overfitting Regularization And Information Criteria
- simpler models tend to be preferred
- this chapter deals with comparing explanatory value of a model and its complexity
- there are two general families:
- regularization
- use a scoring mechanism like information criteria
- this chapter introduces information theory to cover these metrics
6.1 The problem with parameters
- we should not just add all variables even if they are not correlated
with each other
- adding parameters will generally improve the fit of the model to the data
- however, they can often predict worse on new data
- this is overfitting
6.1.1 More parameters always improve fit
- overfitting: a model learns to much from a sample
- for an example, we will use fake data on average brain volumes and body mass of hominid species
sppnames <- c("afarensis", "africanus", "habilis", "boisei",
"rudolfensis", "ergaster", "sapiens")
brainvolcc <- c(438, 452, 612, 521, 752, 871, 1350)
masskg <- c(37.0, 35.5, 34.5, 41.5, 55.5, 61.0, 53.5)
d <- tibble(species=sppnames, brain=brainvolcc, mass=masskg)
- we will use
lm()instead ofquap()for this part of the lesson as it is a bit faster and easier to use- the main points will still apply
- we will build increasingly complex models of this data
- the simplest is below, modelling brain volume of body size
$$ v_i \sim \text{Normal}(\mu_i, \sigma) $$ $$ \mu_i = \alpha + \beta_1 m_i $$
- this model is fit below and the $R^2$ is calculated.
m6_1 <- lm(brain ~ mass, data = d)
1 - var(resid(m6_1)) / var(d$brain) # R-squared
#> [1] 0.490158
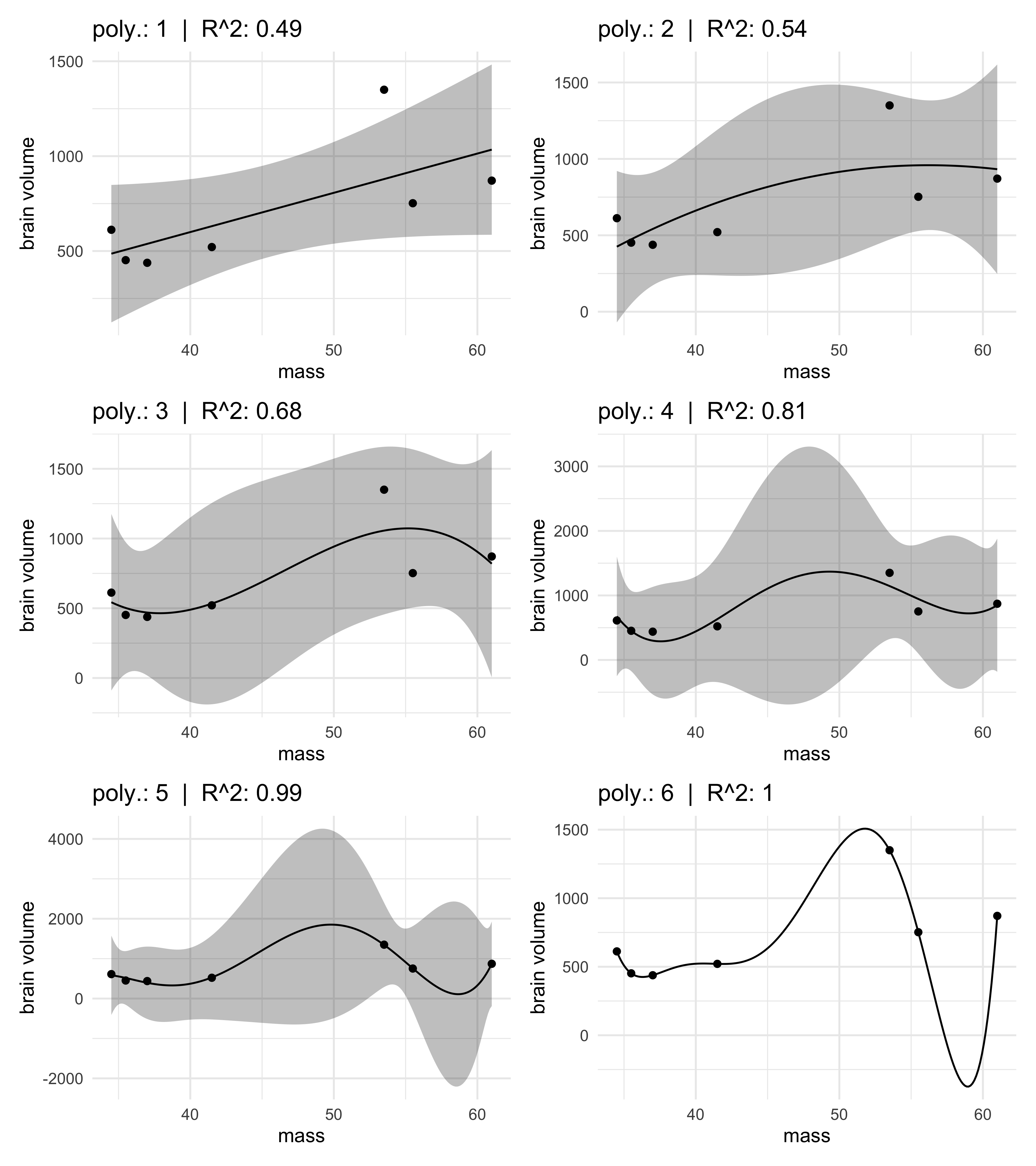
- consider 5 more models, each more complex with higher degree
polynomials
- below is the formula for the 2nd-degree polynomial
$$ v_i \sim \text{Normal}(\mu_i, \sigma) $$ $$ \mu_i = \alpha + \beta_1 m_i + \beta_2 m_i^2 $$
m6_1 <- lm(brain ~ mass, data = d)
m6_2 <- lm(brain ~ mass + I(mass^2), data = d)
m6_3 <- lm(brain ~ mass + I(mass^2) + I(mass^3), data = d)
m6_4 <- lm(brain ~ mass + I(mass^2) + I(mass^3) + I(mass^4), data = d)
m6_5 <- lm(brain ~ mass + I(mass^2) + I(mass^3) + I(mass^4) + I(mass^5), data = d)
m6_6 <- lm(brain ~ mass + I(mass^2) + I(mass^3) + I(mass^4) + I(mass^5) + I(mass^6), data = d)
- all of the fit models are shown against the original data, below
- the 5th order model has an $R^2 = 0.99$ and the 6th-order is 1
- the 6th order goes through every point
- there were enough parameters to assign one to each point of the data
fitted_plot <- function(fit, idx, ...) {
mass_seq <- data.frame(mass = seq(min(fit$model$mass), max(fit$model$mass),
length.out = 200))
predict(fit, newdata = mass_seq, interval = "confidence") %>%
cbind(mass_seq) %>%
ggplot() +
geom_ribbon(aes(x = mass, ymin = lwr, ymax = upr),
alpha = 0.3, color = NA) +
geom_line(aes(x = mass, y = fit)) +
geom_point(data = fit, aes(x = mass, y = brain)) +
labs(title = glue("poly.: {idx} | R^2: {round(glance(fit)$r.squared, 2)}"),
x = "mass", y = "brain volume")
}
list(m6_1, m6_2, m6_3, m6_4, m6_5, m6_6) %>%
purrr::imap(fitted_plot) %>%
patchwork::wrap_plots(ncol = 2)
#> Warning in qt((1 - level)/2, df): NaNs produced
#> Warning in max(ids, na.rm = TRUE): no non-missing arguments to max; returning -
#> Inf
6.1.2 Too few parameters hurts, too
- too few parameters means the model has learned too little from the data
6.2 Information theory and model performance
(My notes on this section are relatively brief and focused on the main points application.)
- must choose a target criterion of model performance
- this can be based on regularization or information criteria
- then must choose a metric
6.2.1 Firing the weatherperson
- accuracy depends on definition of the target
- two dimensions to accuracy:
- cost-benefit analysis: how much does it cost to be wrong?
- accuracy in context: how much can the model improve prediction?
- two dimensions to accuracy:
- we will use a weather prediction for example
- two weathermen make the following predictions for the probability of rain over the next 10 days in the same city
- the bottom row is the observed outcome
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pred 1 | 1 | 1 | 1 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Pred 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Obs | R | R | R | S | S | S | S | S | S | S |
- the second prediction was correct more often than the first
- a higher hit rate (rate of correct predictions)
6.2.1.1 Costs and benefits
- if we weight being caught in the rain as -5 and carrying an umbrella
as -1:
- prediction 1 score: -7.2
- prediction 2 score: -15
6.2.1.2 Measuring accuracy
- hit rate is not the only measure of accuracy
- perhaps compute the probability of predicting the exact sequence of
days:
- compute the probability of a correct prediction for each day
- multiply each probability together to get the joint probability of correctly predicting the sequence
- this is the joint likelihood for Bayes’ theorem
- prediction 1 had a probability of $1^3 \times 0.4^7 \approx 0.005$
and prediction 2 had $0^3 \times 1^7 = 0$
- the 2nd prediction had no chance of being correct
- the 2nd prediction had a high average probability of being correct, it had a bad join probability of being correct
- the joint probability is the likelihood in Bayes’ theorem
- the number of ways each event (sequence of rain and shine) could happen
- now need to find a way to measure how incorrect the prediction is
- needs to account for how hard getting the correct prediction is
6.2.2 Information and uncertainty
- How much is our uncertainty reduced by learning an outcome?
- how much is learned when we actually observe the day’s weather
- need a measure of uncertainty
- information: the reduction in uncertainty derived from learning an outcome
- need a way to quantify uncertainty inherent in a probability
distribution
- use information entropy: “The uncertainty contained in a probability distribution is the average log-probability of an event.”
$$ H(p) = -E \log(p_i) = \ \sum_{i=1}^{n} p_i \log(p_i) $$
6.2.3 From entropy to accuracy
- now need a measure of how far a model is from the target: divergence
- divergence: “The additional uncertainty induced by using
probabilities from one distribution to describe another
distribution.”
- also known as Kullback-Leibler divergence (K-L divergence)
- suppose the probabilities for two events are $p_1 = 0.3$,
$p_2 = 0.7$
- if we instead believe these events have the probabilities $q_1 = 0.25$, $q_2 = 0.75$
- measure how much additional uncertainty has been introduced by using $q = {q_1, q_2}$ to estimate $p = {p_1, p_2}$?
$$ D_\text{KL}(p, q) = \sum_i p_i (\log(p_i) - \log(q_i)) = \sum_i p_i \log(\frac{p_i}{q_i}) $$
- “the divergence is the average difference in log probability between
the target (p) and model (q)”
- the difference between the entropy of the target $p$ and the cross entropy from using $q$ to predict $p$
6.2.4 From divergence to deviance
- the above sections established the distance of a model from the target using K-L divergence
- the remaining is to see how to estimate this divergence in a statistical model
- so far, we have been comparing the model to the truth: compare true
probability $p$ to estimated probability $q$
- the truth is not known in reality, though
- instead, we can compare two estimated $q$ and $r$, which
causes the real probability $p$ to be subtracted out
- cannot how far from the truth either model is, but we can tell which is further
- we end up with a relative model fit as an estimate of K-L
divergence
- we measure a model’s deviance:
$$ D(q) = -2 \sum_i \log(q_i) $$
- the deviance of the estimates we have modeled in this course so far
can be calculated:
- the $q$-values: use the MAP estimates to compute a log-probability of the observed data for each row
- sum and multiply by $-2$
- in R, this can be done by the
logLik()function
-2 * logLik(m6_1) # deviance
#> 'log Lik.' 94.92499 (df=3)
6.2.5 From deviance to out-of-sample
- flaw with deviance: it will always improve with model complexity
- just like $R^2$
- the deviance on new data is really what we want
6.3 Regularization
- can prevent overfitting by adding a regularization prior to a
$\beta$ coefficient
- requires tuning to prevent both overfitting and underfitting
- a uniform or other broad prior may allow the model fitting algorithm to stray to extreme
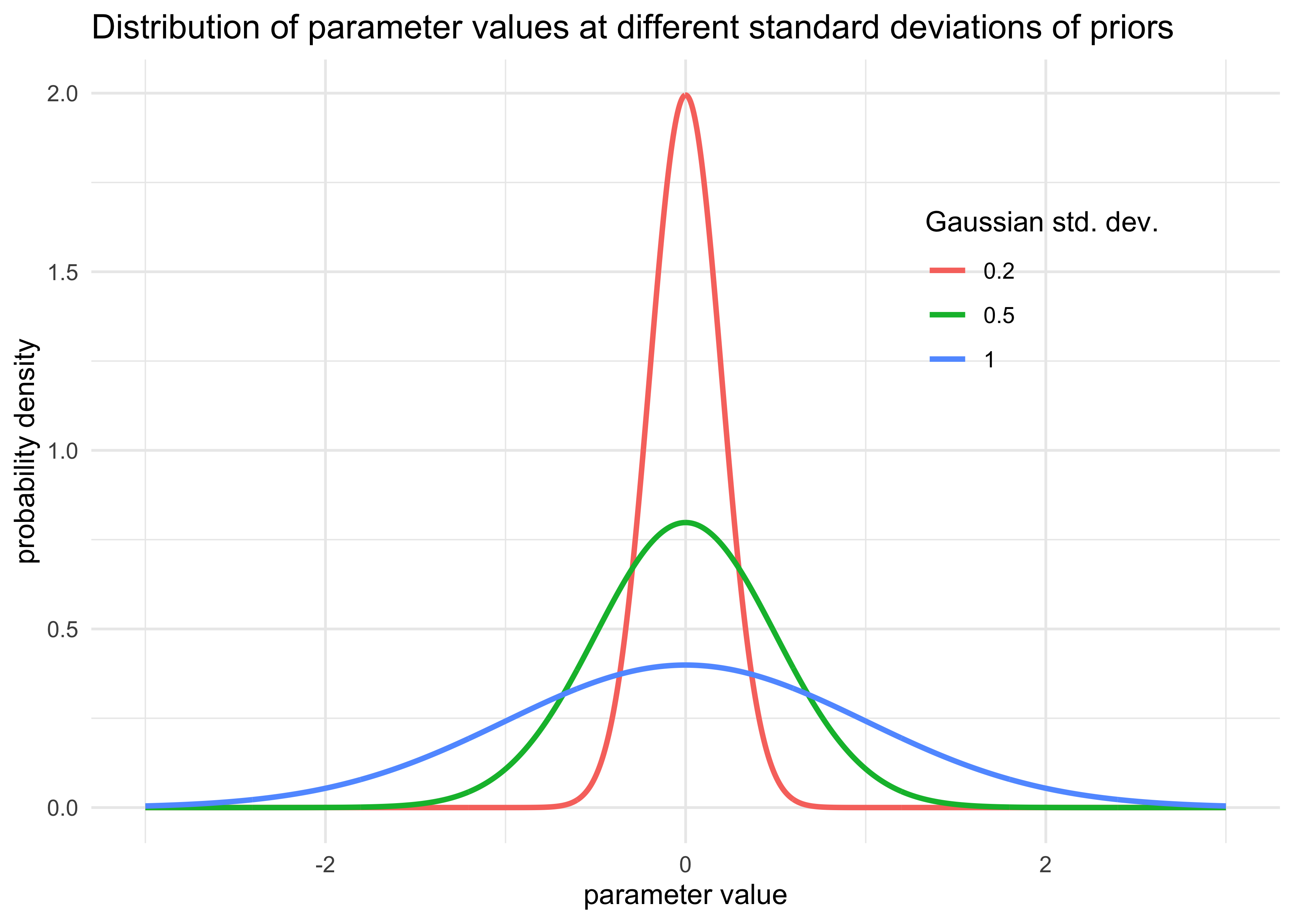
- for example, consider the following Gaussian model (where $x$ is
standardized)
- the prior for $\alpha$ is effectively uninformative
- the prior for $\beta$ is very strict
$$ y_i \sim \text{Normal}(\mu_i, \sigma) $$ $$ \mu_i = \alpha + \beta x_i $$ $$ \alpha \sim \text{Normal}(0, 100) $$ $$ \beta \sim \text{Normal}(0, 1) $$ $$ \sigma \sim \text{Uniform}(0, 10) $$
tibble(x = list(seq(-3, 3, length.out = 1e3)),
std_dev = c(1, 0.5, 0.2)) %>%
unnest(x) %>%
mutate(prob_density = map2_dbl(x, std_dev, ~ dnorm(.x, mean = 0, sd = .y))) %>%
ggplot(aes(x = x, y = prob_density, color = factor(std_dev))) +
geom_line(size = 1) +
theme(legend.position = c(0.8, 0.7)) +
labs(x = "parameter value",
y = "probability density",
title = "Distribution of parameter values at different standard deviations of priors",
color = "Gaussian std. dev.")
- the amount of regularization can be dewtermined using cross validation
6.4 Information criteria
- Akaike information criterion provides an estimate of out-of-sample
deviance:
- $p$ is the number of free parameters to be estimated
$$ \text{AIC} = D_\text{train} + 2p $$
- AIC’s approximation is reliable when:
- the priors are flat
- the posterior distribution is approximately multivariate Gaussian
- the sample size $N$ is much larger than the number of parameters $k$
- because we rarely want to use flat priors, use DIC and WAIC more
frequently:
- Deviance Information Criterion (DIC) accomidates informative priors but still assumes the posterior is multivariate Gaussian and $N \gg k$
- Widely Applicable Information Criterion (WAIC) is more general
- makes no assumptions about the posterior
6.4.1 DIC
- Deviance Information Criterion (DIC)
- it is aware of informative priors
- by using the posterior distributions to calculate the deviance
- assumes a multivariate Gaussian posterior distribution (like AIC)
- if a parameter in the posterior is highly skewed, DIC (and AIC) will give poor estimates
6.4.2 WAIC
- Widely Applicable Information Criterion (WAIC)
- does not require a multivariate Gaussian posterior
- is often more accurate than DIC
- however, there are some models where defining WAIC is too difficult
- particularly where the points are not independent (line in time-series analysis)
- it operates point-wise
- uncertainty in prediction is considered for each point in the data
6.4.3 DIC and WAIC as estimates of deviance
6.5 Using information criteria
- this section discusses model comparison and model averaging:
- model comparison: using DIC/WAIC in combination with posterior predictive checks to each model
- model averaging:using DIC/WAIC to construct a posterior
predictive distribution that uses what we know about relative
accuracy of the models
- “prediction averaging,” not averaging the estimates of multiple models
6.5.1 Model comparison
- use the primate milk data for an example
- remove rows with missing information
data("milk")
d <- milk[complete.cases(milk), ] %>% janitor::clean_names()
d$neocortex <- d$neocortex_perc / 100
- predict kilocalories per gram using neocortex size and the lagarithm
of mass of the mother
- fit 4 different models with different combinations of these predictors
- also restrict the standard deviation of the final Gaussian
$\sigma$ to be positive
- estimate the logarithm of $\sigma$ and exponentiate it in the likelihood function
- all priors were left as flat
a_start <- mean(d$kcal_per_g)
sigma_start <- log(sd(d$kcal_per_g))
# Model kcal with no predictors (just an intercept)
m6_11 <- quap(
alist(
kcal_per_g ~ dnorm(a, exp(log_sigma))
),
data = d,
start = list(a = a_start,
log_sigma = sigma_start)
)
# Model kcal with neocortex
m6_12 <- quap(
alist(
kcal_per_g ~ dnorm(mu, exp(log_sigma)),
mu <- a + bn*neocortex
),
data = d,
start = list(a = a_start,
bn = 0,
log_sigma = sigma_start)
)
# Model kcal with log(mass)
m6_13 <- quap(
alist(
kcal_per_g ~ dnorm(mu, exp(log_sigma)),
mu <- a + bm*log(mass)
),
data = d,
start = list(a = a_start,
bm = 0,
log_sigma = sigma_start)
)
# Model kcal with neocortex and log(mass)
m6_14 <- quap(
alist(
kcal_per_g ~ dnorm(mu, exp(log_sigma)),
mu <- a + bn*neocortex + bm*log(mass)
),
data = d,
start = list(a = a_start,
bn = 0,
bm = 0,
log_sigma = sigma_start)
)
6.5.1.1 Comparing WAIC values
- can measure WAIC using the
WAIC()function from the ‘rethinking’ package:- first value is the WAIC
lppdandpWAICare used to calculate WAICseis the standard error of the estimate from the sampling process
WAIC(m6_14)
#> WAIC lppd penalty std_err
#> 1 -15.39206 12.33699 4.640955 7.612751
- get WAIC for all models
- the “weight” is roughly “an estiamte of the probability that the model will make the best predictions on new data, conditional on the set of models considered”
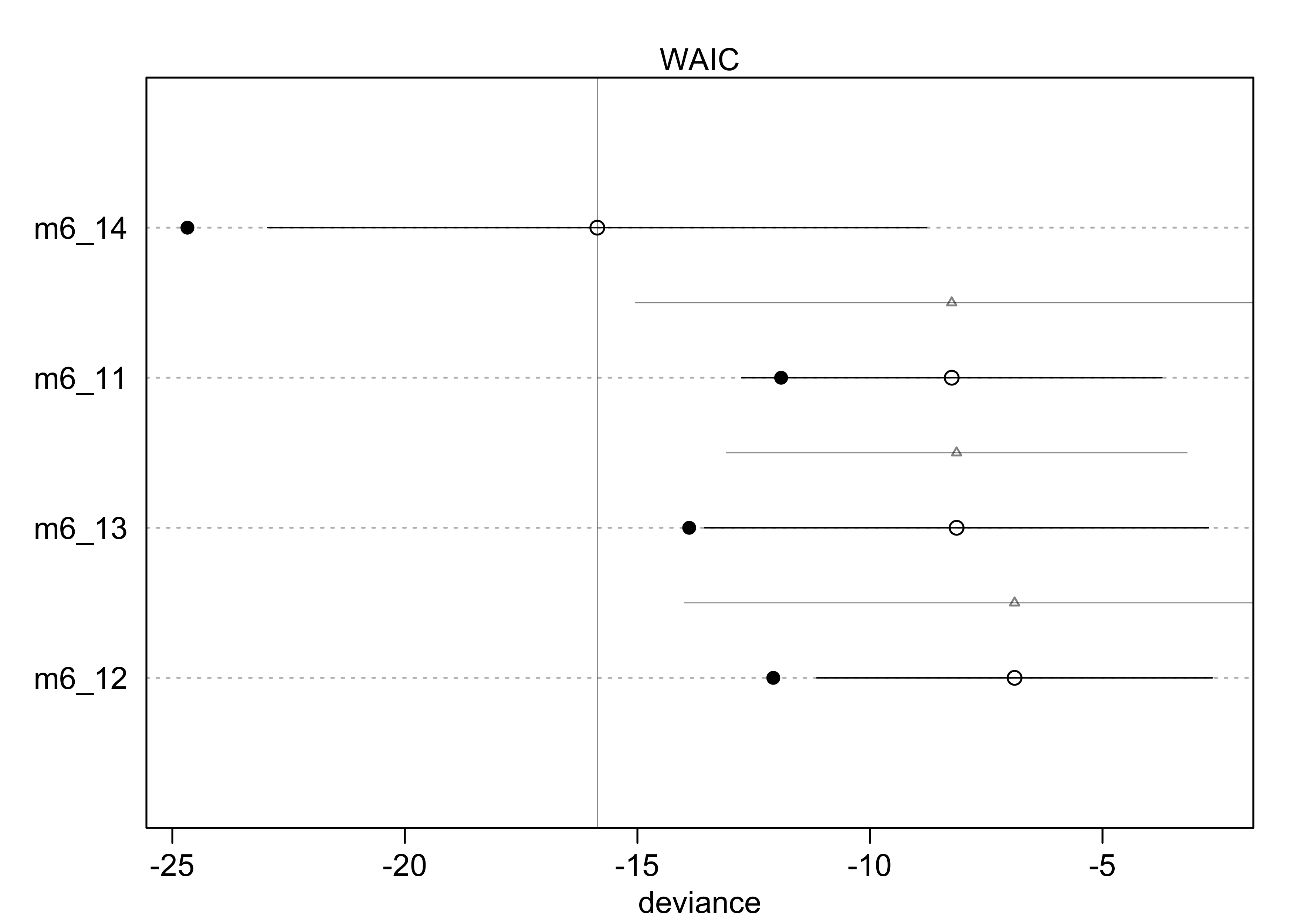
milk_models <- compare(m6_11, m6_12, m6_13, m6_14)
milk_models
#> WAIC SE dWAIC dSE pWAIC weight
#> m6_14 -15.862631 7.081823 0.000000 NA 4.406564 0.94839376
#> m6_11 -8.242263 4.517978 7.620368 6.802299 1.834151 0.02100133
#> m6_13 -8.136854 5.418315 7.725778 4.954064 2.874894 0.01992312
#> m6_12 -6.890172 4.251610 8.972459 7.101033 2.594300 0.01068179
- can plot the WAIC values and distributions
- unfilled points are the WAIC
- the SE of the difference between each WAIC and the top-ranke WAIC are the grey triangles
plot(milk_models, SE = TRUE, dSE = TRUE)
6.5.1.2 Comparing estimates
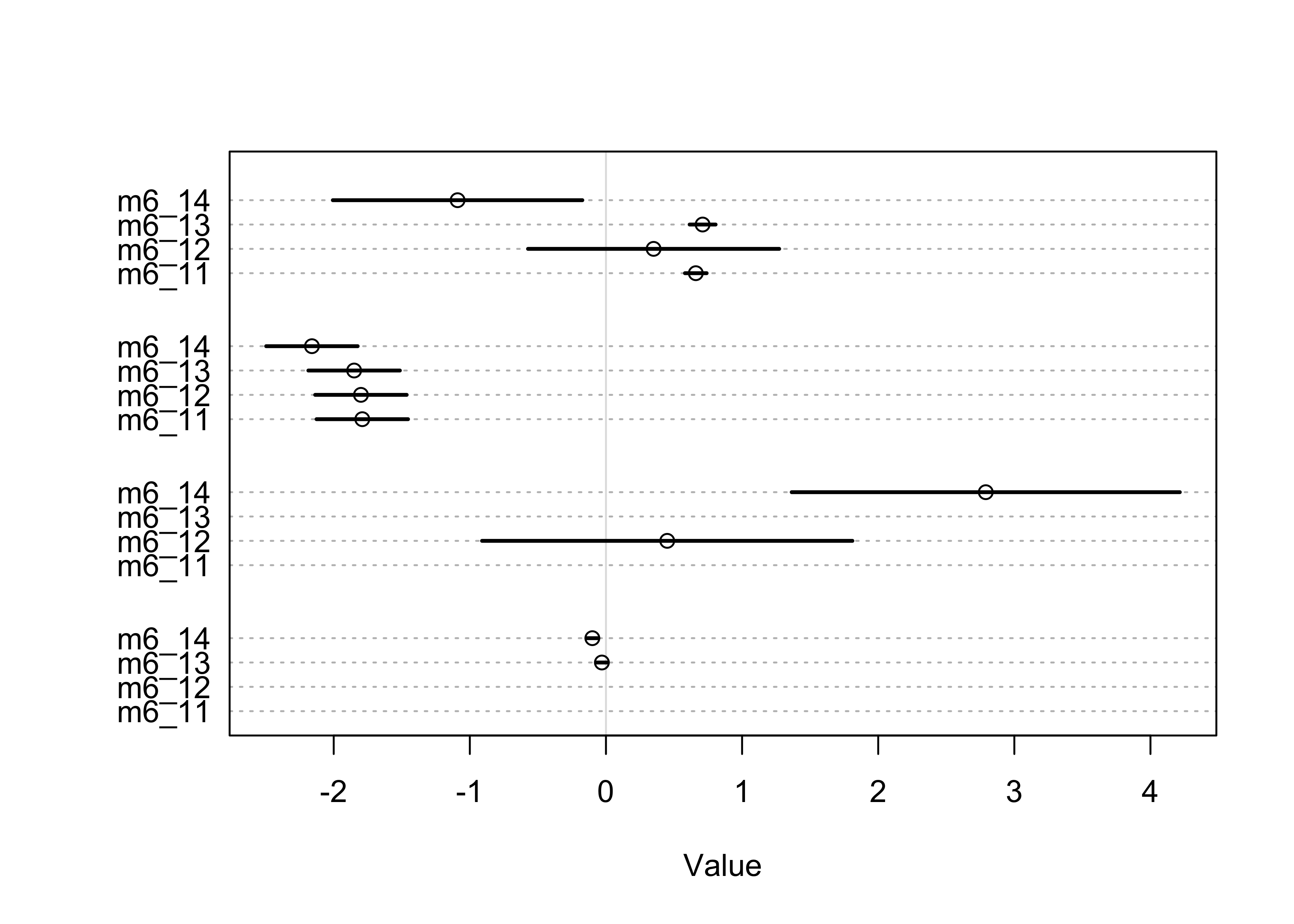
- generally useful to compare parameter estimates of models
- can help to understand the WAIC values
- helps to understand the parameters to see if they remain stable across models
coeftab(m6_11, m6_12, m6_13, m6_14)
#> m6_11 m6_12 m6_13 m6_14
#> a 0.66 0.35 0.71 -1.09
#> log_sigma -1.79 -1.80 -1.85 -2.16
#> bn NA 0.45 NA 2.79
#> bm NA NA -0.03 -0.10
#> nobs 17 17 17 17
plot(coeftab(m6_11, m6_12, m6_13, m6_14))
6.5.2 Model averaging
- we want to use the weights from WAIC to generate predictions
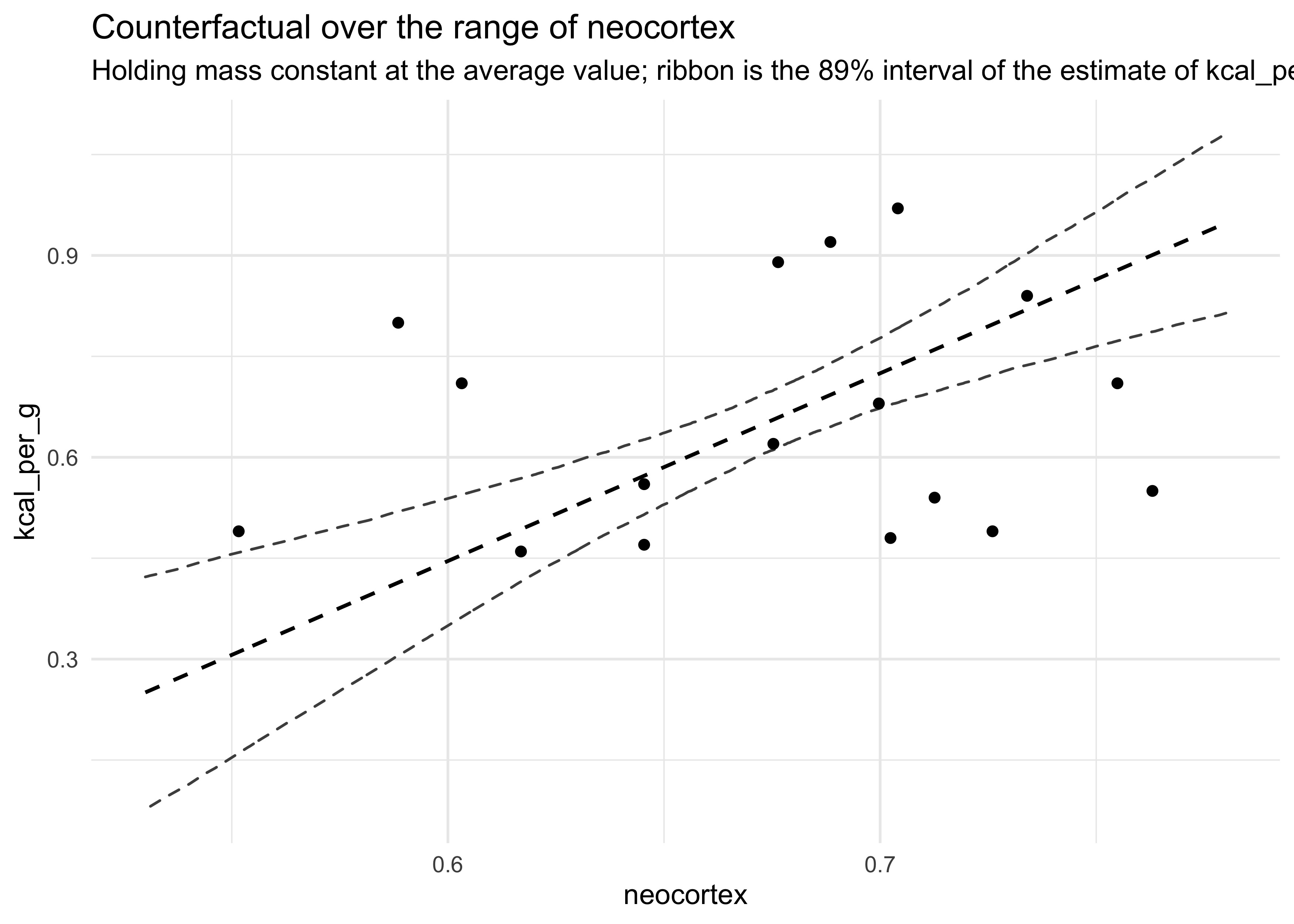
- to review, start with simulating and plotting counterfactual
predictions for
m6_14on the range of neocortex
nc_seq <- seq(0.53, 0.78, length.out = 1e3)
d_predict <- tibble(
kcal_per_g = 0,
neocortex = nc_seq,
mass = 4.5
)
pred_m6_14 <- link(m6_14, data = d_predict)
mu <- apply(pred_m6_14, 2, mean)
mu_pi <- apply(pred_m6_14, 2, PI)
d_predict %>%
mutate(mu_mean = mu) %>%
bind_cols(pi_to_df(mu_pi)) %>%
ggplot() +
geom_ribbon(aes(x = neocortex, ymin = x5_percent, ymax = x94_percent),
fill = NA, color = "grey30", lty = 2) +
geom_line(aes(x = neocortex, y = mu_mean), lty = 2, size = 0.7) +
geom_point(data = d, aes(x = neocortex, y = kcal_per_g)) +
labs(x = "neocortex",
y = "kcal_per_g",
title = "Counterfactual over the range of neocortex",
subtitle = "Holding mass constant at the average value; ribbon is the 89% interval of the estimate of kcal_per_g")
- now we can compute and add model-averaged posterior predictions:
- compute WAIC for each model
- compute the weight for each model
- compute linear model and simulated outcomes for each model
- combine these values into an ensemble of predictions, using the model weights as proportions
- this is automated by the
ensemble()function from ‘rethinking’- it uses
link()andsim()to do the above process
- it uses
milk_ensemble <- ensemble(m6_11, m6_12, m6_13, m6_14, data = d_predict)
mu_ens <- apply(milk_ensemble$link, 2, mean)
mu_pi_ens <- apply(milk_ensemble$link, 2, PI)
d_predict %>%
mutate(mu_mean = mu,
ensemble_mu = mu_ens) %>%
bind_cols(pi_to_df(mu_pi) %>% set_names(c("m6_14_low", "m6_14_hi"))) %>%
bind_cols(pi_to_df(mu_pi_ens) %>% set_names(c("ens_low", "end_hi"))) %>%
ggplot(aes(x = neocortex)) +
geom_ribbon(aes(ymin = m6_14_low, ymax = m6_14_hi),
fill = NA, color = "grey30", lty = 2) +
geom_line(aes(y = mu_mean), lty = 2, size = 0.7) +
geom_ribbon(aes(ymin = ens_low, ymax = end_hi),
fill = "black", color = NA, alpha = 0.2) +
geom_line(aes(y = ensemble_mu), lty = 1, size = 0.7) +
geom_point(data = d, aes(y = kcal_per_g)) +
labs(x = "neocortex",
y = "kcal_per_g",
title = "Counterfactual over the range of neocortex",
subtitle =
"Holding mass constant at the avg.; dashed ribbon is the 89% interval of the estimate of kcal_per_g.
The shaded region and solid line are estimates using the ensemble approach.")
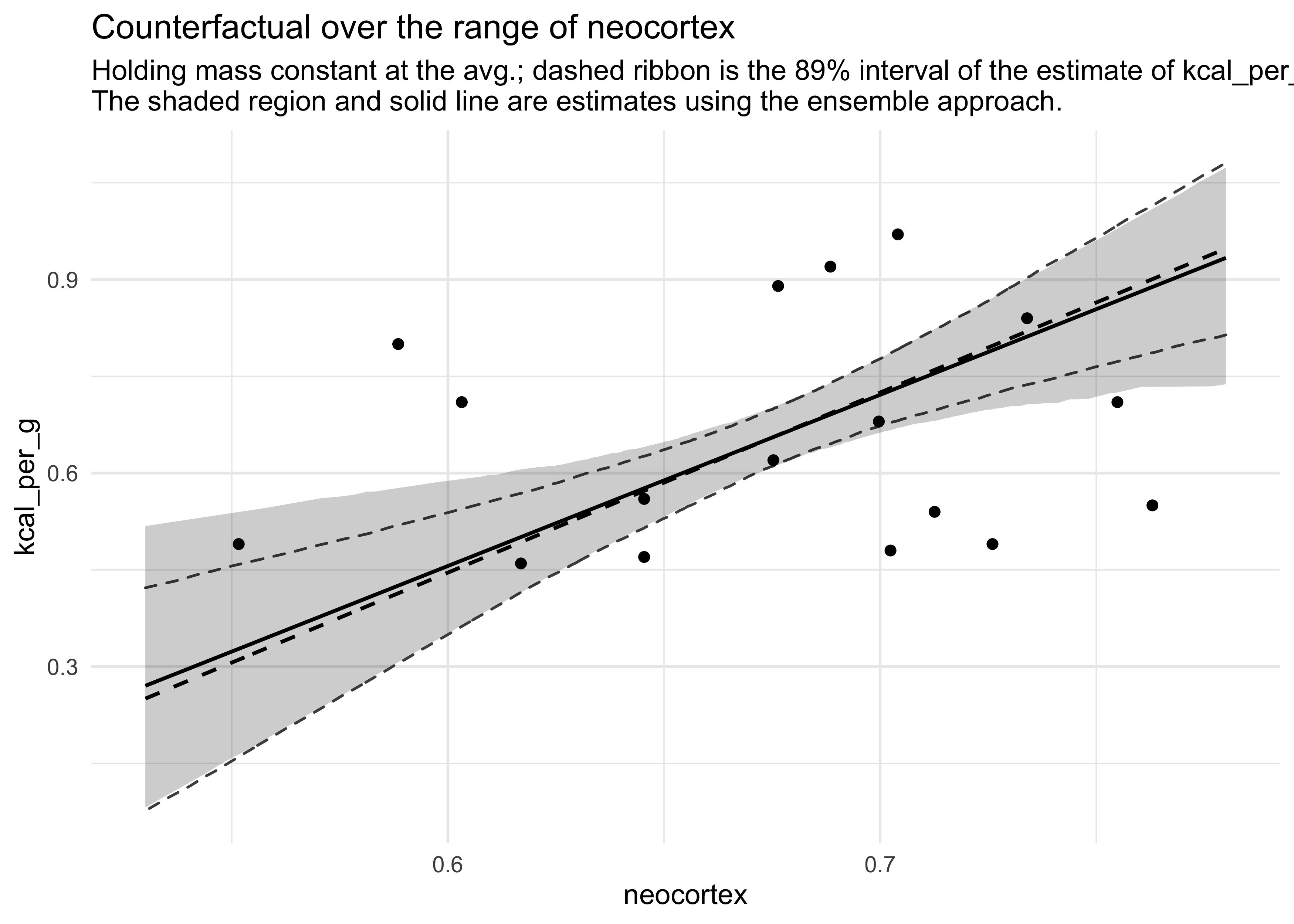
- model averaging is a conservative approach
- it communicates model uncertainty
- will never make a predictor variable appear more influential than it already does in any single model